![]()
Immune System Recognition and Identification of the "Right" Antigens for Active Immunotherapy
Immunotope’s technology platform is based on a deep understanding of the natural response of the human immune system to the presence of disease. We use mass spectrometry and differential analysis to identify panels of the 'Right Antigens' that comprise the immunoproteome; these are naturally presented, Major Histocompatibility Complex (MHC)-associated, disease-specific ‘signature’ antigens displayed on the surfaces of tumor and infected cells. These antigens are potent targets for diseased cell destruction by cytotoxic T lymphocytes.
All of the body’s cells continuously report their physiological status to the immune system by diverting a small proportion of intracellular and cell surface proteins into the Major Histocompatibility Complex (MHC) pathway, in which the proteins are broken up into 9 to 11 amino acid fragments (antigens) and associated with MHC proteins. The MHC proteins transport these peptide antigens to the cell surface and ‘display’ them to surveillance by cytotoxic T lymphocytes (CTL), which are specialized white blood cells whose function is to circulate throughout the body and recognize and destroy cells that display an antigen ‘signature’ characteristic of disease. The vast majority of displayed fragments in this signature, the immunoproteome, are ‘self’, or normal fragments, however on diseased cells a small proportion of peptides are ‘altered self’ (e.g. cancer cell) or ‘foreign’ (infected cell). Tumor and infected cells have distinctly different peptide ‘antigen signatures’ from normal, healthy cells. If activated properly with an immunotherapeutic vaccine, T cells can distinguish ‘self’ from ‘altered self’ with extremely high fidelity and sensitivity- as little as one peptide per cell- and specifically attack and kill cells displaying altered self or foreign antigen signatures.
The
key to developing successful immunotherapeutic vaccines is to
identify the antigens that are presented only in diseased
cell signatures and not in healthy cell signatures.
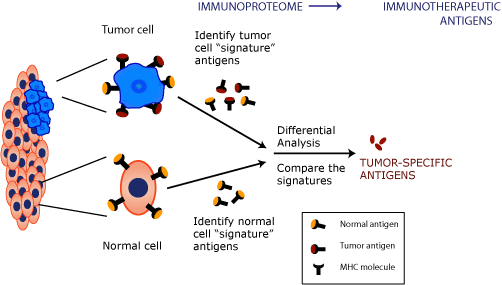
CTL recognize antigenic peptides only in the context of their association with MHC molecules. Therefore, isolating MHC-associated peptide antigens that are presented on diseased cells enables us to identify panels of T cell-target epitopes that originate from the tumor or the pathogen, and therefore the most immunologically and clinically relevant candidates for immunotherapeutics development. In this process, the proteins from which the peptide fragments originated are also identified. These proteins are potential vaccine, diagnostic and antibody target candidates. The identification of antigens that are only displayed on diseased cells, and not on healthy cells is important for vaccine development, as it avoids the problem of autoimmunity.
A key advantage of immunoproteomics compared to the use of predictive algorithms, or ‘reverse vaccinology’, is that each candidate is a known immune target, and for the discovery process, prior identification of the source protein of the presented antigen is not required. Direct identification of endogenous MHC-associated peptides is very powerful because it identifies multiple “shared” antigens in the same tumor type from several different patients as well as antigens common or shared among different tumor types.
Proof of concept studies for Immunotope’s technology have been successfully conducted for melanoma, prostate cancer and Mycobacterium tuberculosis, demonstrating its broad application to cancers and infectious diseases.
.....................................................................................................................................................................
Clinical and Preclinical Programs | Science & Technology | Careers
Business Development |
Services
| Management and
Boards | News
and Media | Contact Us |
Home